This person is not a real SYMTUZA® patient.
Why switch to SYMTUZA®?
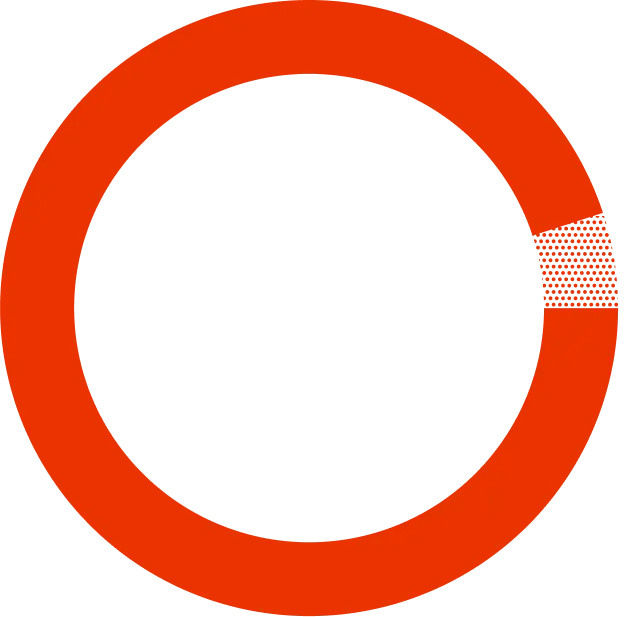
who switched to SYMTUZA® stayed undetectable (<50 copies/mL) after 48 weeks.*
At the start of the trial, 763 of the patients switched to SYMTUZA®, while the remaining 378 continued on their previously prescribed treatment. The final measure of the study was to see the number of patients who remained undetectable after 48 weeks after switching to SYMTUZA®.
1% of patients taking SYMTUZA® stopped participating in the trial due to side effects compared to 1% of patients on their current treatment who stopped participating.
The most common drug-related side effects occurring in at least 2% of patients on SYMTUZA® were diarrhea and bone weakness. These are not the only side effects of SYMTUZA®.
Learn more*There were patients who stopped the study for other reasons. 1% of patients didn’t stay undetectable (viral load <50 copies/mL), and among patients in the study who didn’t switch to SYMTUZA®, 94% stayed undetectable versus 1% who did not. The study looked at 1,141 patients already being treated for HIV and who were undetectable (<50 copies/mL) for at least 6 months.
Take steps to switch treatment.
If you and your healthcare provider decide that switching to SYMTUZA® is right for you, it's important to keep an open line of communication. Ask questions and voice your concerns as you make the switch. Remember: You have a say in your treatment decisions.